During the first half of July, confusion over the need for a third dose of a COVID-19 vaccine began to mount after the vaccine giant Pfizer-BioNTech announced its intention to seek authorization for a booster shot in the United States. Adding to the confusion was Israel’s decision to offer a third dose of the Pfizer vaccine to those adults who were vulnerable to infection caused by the Delta variant.
Efforts to administer the third dose of a two-dose COVID-19 vaccine first started in France, with an increasing number of immunocompromised citizens seeking to trigger an antibody response. A study conducted by a team of French researchers back in June reported that organ transplant recipients who had been administered dose three of the Pfizer COVID-19 vaccine showed a 68% increase in antibodies within a four-week span. When compared to organ transplant recipients who received two doses of the Pfizer COVID-19 vaccine, the increase in antibodies was as low as 40%.
The list of those immunocompromised in the population involves people with certain cancers, chronic liver disease, kidney failure, organ transplants, and those on dialysis. It also involves being immunocompromised due to certain drugs which include methotrexate, Rituxan, and various steroids. But countries like France have decided to make these booster vaccines available to individuals aged over 75 and health care workers aged over 50 as well.
While administration of the third dose began as a cautionary measure for those vulnerable, it has led to questions over the efficacy of the current doses. In May, two doses of the Pfizer vaccine stood at an efficacy rate of 88% against the Delta variant. Subsequent research by teams in Canada and Scotland placed its efficacy at 87% and 75% respectively. Surprisingly, the efficacy rate was reported as 64% in a study conducted by Israel’s Ministry of Health which they cited as a reason for administering dose three of the vaccine.
It’s possible for the range to be this wide, after all a single study cannot accurately pinpoint what the effectiveness of the vaccine might be. But vaccine makers like Pfizer in a statement claimed that administering dose three would help establish the highest protective efficacy against variants like the Delta.
“While protection against severe disease remained high across the full 6 months, a decline in efficacy against symptomatic disease over time and the continued emergence of variants are expected,” the company said.
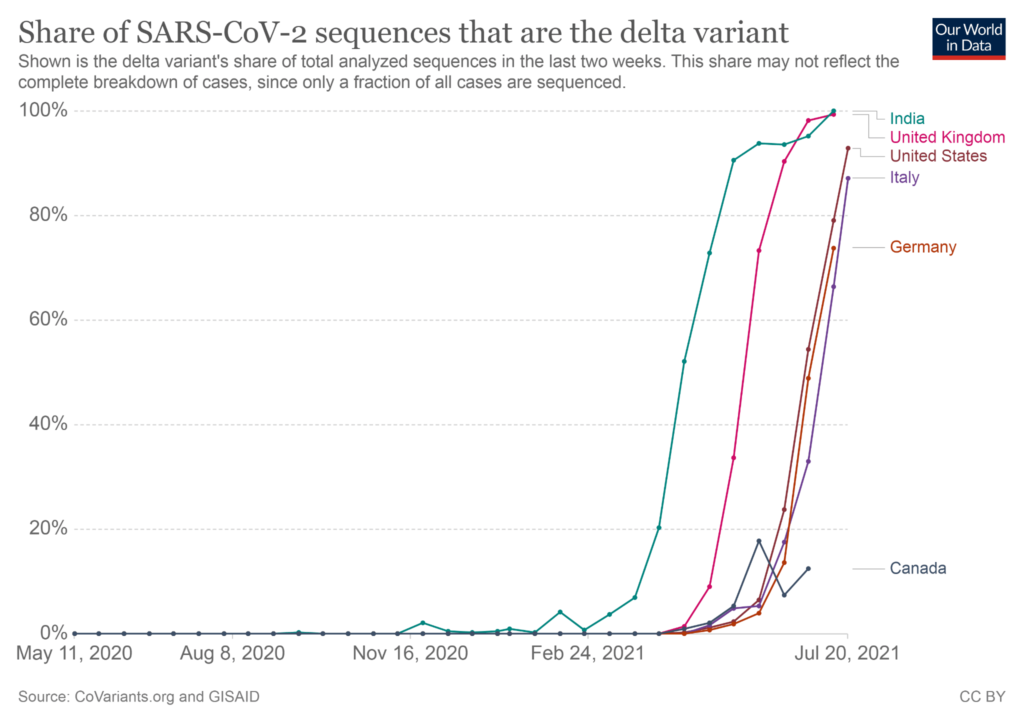
On the other hand, vaccine experts have dismissed Pfizer’s claims reiterating that the Pfizer, Moderna, and the Johnson & Johnson vaccines provide strong protection against COVID-19. Even the Centers for Disease Control and Prevention and the Food and Drug Administration were quick to state that“Americans who have been fully vaccinated do not need a booster shot at this time.”
While it might take another 12 months for the booster shots to be available in the United States, let alone be approved, we are now forced to think of countries struggling to provide even the first dose to their populations. For example, a number of African countries are yet to start mass vaccination drives.
Tedros Adhanom, the director general of the World Health Organization in a statement condemned vaccine manufacturers like Pfizer for encouraging wealthy nations to purchase booster shots when a large number of countries were struggling to gain access to the first dose. “The global gap in vaccine supply is hugely uneven and inequitable,” he added.
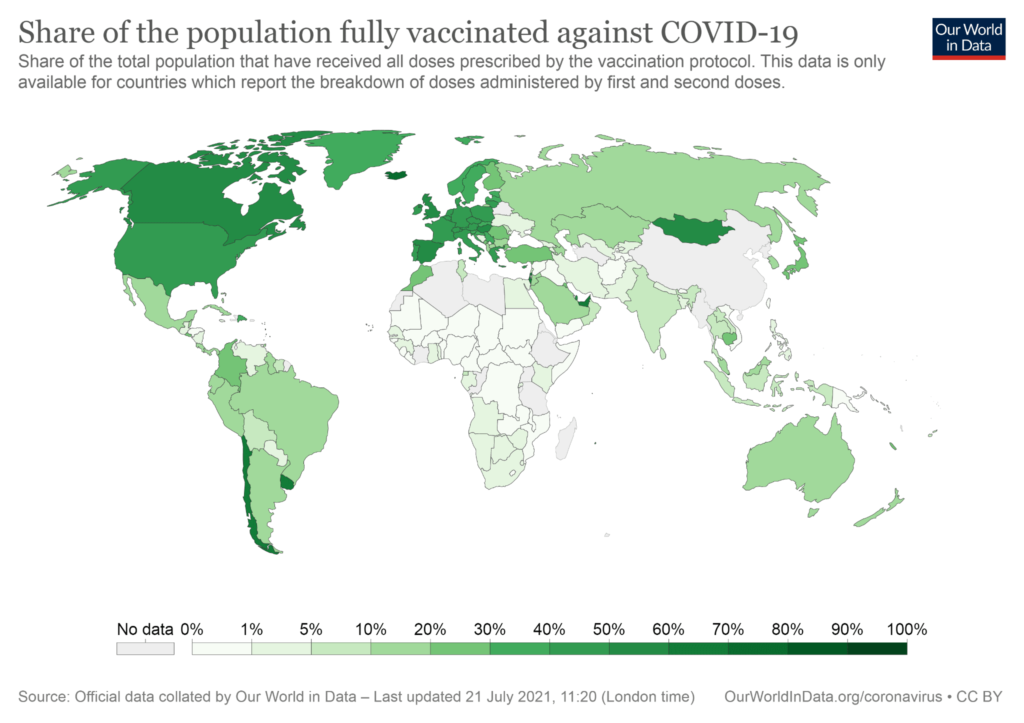
India is on a similar footing with just over 5% of the entire population fully vaccinated. The country’s vaccination drive was off to a promising start back in January, but with vaccine shortages and a second wave incapacitating the country in April, the drive has now slowed down significantly.
Back in June, Moderna first announced the Indian Government’s intention to import doses of their novel mRNA vaccine for administration in the country. However, it doesn’t seem like these vaccines will be available soon since talks are still ongoing with both Moderna and Pfizer regarding various issues including indemnity waivers. “We are holding discussions. It is a process of negotiation and dialogues. We are trying to get a solution on contractual and commitments issues,” Dr. VK Paul, Member (Health), NITI Aayog told ANI.
As vaccine shortages continue to plague a number of countries, the race to beat newly emerging variants continues. As opposed to getting three doses of the vaccine, research has shown time and again that fully vaccinated individuals are highly protected against emerging variants. For example, a study by researchers in France reported that two doses of the Pfizer or AstraZeneca vaccine showed an immune boost that was enough to neutralize the Delta variant.
What’s crucial here is getting more of the world immunized before new variants emerge. Not only is the growing vaccine gap and unequal distribution of doses unethical, but it also prolongs the pandemic.
Written by- Shalet Jeevita Serrao
Edited by- Nanditha N Menon
